DIAMOND
ALKALI/SHAMROCK
PAINESVILLE
WORKS
The Diamond's Painesville Works and
the Fairport, Painesville & Eastern were closely connected to each other throughout
their histories: both the plant and the railroad were constructed and began
operations at the same time; in the decades following their creation, as the
Works grew, so did the FP&E; and unfortunately, when the Works closed the
FP&E was not far behind it in fading into history. With the plant being so closely tied to the
railroad—not to mention being the railroad's largest customer by far for
64 years of its 72-year operating existence—I believe it is a necessity to
discuss in some detail the Diamond's Painesville Works as part of any
discussion about the FP&E.
Below
is some information I've gathered about the Diamond, and I've organized into
three sections: a historical background of the Diamond Alkali/Shamrock
Corporation in general, a description of some of the manufacturing processes at
the Works circa 1956, and a chronology of the different portions of the Works.
Historical Background
I have found two very good general histories of
the Diamond Alkali/Shamrock Corporation; both are entries from editions of the International
Directory of Company Histories, and both can be read on the internet. From the International Directory of
Company Histories: Volume 7 there is an entry for Maxus Energy Corporation
which, as the successor to the Diamond Shamrock's oil production and
exploration divisions, includes a good amount of historical background
information on the Diamond (click here
to read the entry). From the International
Directory of Company Histories: Volume 31 there is an entry for Ultramar
Diamond Shamrock Corporation which, as the successor to the Diamond Shamrock's
oil refining and marketing divisions, includes an even larger amount of
historical background information on the Diamond (click here
to read the entry). Though the
information in these articles covers the same territory, they are not
identical, so it is very worthwhile to read both of them.
For those who prefer
something shorter I wrote the following brief version of the Diamond's history
based on the two articles linked above plus excerpts from the books Glass: Shattering Notions, Strategies for
Declining Businesses, Encyclopedia of Chemical Processing and Design:
Volume 51, and Applied Industrial Economics.
Diamond Alkali was founded in 1910 by three
glass-making companies (Macbeth-Evans Glass Co. of Pittsburgh, C.L. Flaccus
Glass Co. of Pittsburgh, and Hazel-Atlas Glass Co. of Wheeling, WV) with the
purpose of manufacturing soda ash—a major raw material in glass
production. The new firm was
incorporated on March 21, 1910 in West Virginia, with the company's
headquarters established in Pittsburgh.
With a capitalization of $1.2 million, a soda ash plant was built in
Painesville Township, Ohio. The plant began operation in 1912; however, it
wasn't until the demand for glass dramatically increased with the start of
World War I that the company's business boomed.
After the war the company expanded into other
product areas: production of bicarbonate of soda began in 1918; in 1924-25 the
plant was expanded to produce calcium carbonates, cement, and coke; and in 1929
production of chlorine was begun.
To ensure the company would not stagnate a
research program was begun in 1936, and in 1942 Diamond Alkali established a
research laboratory. One result of the
company's 1936 research program was the production of magnesium oxide. When magnesium became an important ingredient
in incendiary bombs during World War II, the Diamond was recruited by the U.S.
government to build and operate a magnesium plant for the Defense Department.
In the decade after World War II the company
continued to expand its product range in Painesville, but also expanded
geographically through acquisitions and new plants—including a second
chlorine/caustic soda plant in Houston (Deer Park). Though some of the acquisitions and new plants
increased the production capability of chemicals the Diamond already made, some
of the newly acquired companies and new facilities had the effect of
diversifying the company's products to include such things as agricultural
chemicals and plastics.
Reflecting the importance of its operations in
Painesville, in 1948 the corporate headquarters was moved from Pittsburgh to
Cleveland. The company continued its
emphasis on research by opening the Diamond Technical Center in Fairport Harbor
in 1951, and by opening the Diamond Research Center in Concord Township in
1961.
In the 1960s Diamond Alkali's expansion
continued with acquisitions of numerous chemical and plastics companies. During this decade the company also created a
specialty chemicals division, expanded production of industrial chemicals and
plastics at its existing plants, and built new chlorine/caustic soda and PVC
plants in Delaware.
Recognizing the current trend in petrochemical
combines—and not wanting to be acquired by a large oil company—Diamond Alkali
made a pre-emptive move by approaching Texas-based Shamrock Oil & Gas with
a merger plan in the mid-1960s; in 1967 the two companies merged to form
Diamond Shamrock Corporation.
In the 1970s Diamond Shamrock continued to
grow—especially in the areas of specialty chemicals, petroleum, and
plastics. Though the company was
becoming more focused on these other areas, industrial chemicals were still
important, and in 1974 construction began on a new chlorine/caustic soda plant
at Houston (Battleground). But despite
the company's continued growth, the original core product of the company, soda
ash, the primary product of the Painesville Works, was in decline.
Though soda ash can be made synthetically from
limestone and salt—as was done at the Painesville Works—it also occurs
naturally. Mining natural soda ash is
mechanically easier than producing synthetic natural soda, uses less energy,
and causes less pollution. However, since
most of the natural soda ash deposits in the U.S. were out west, while most of
the soda ash customers were located east of the Mississippi (along with the
synthetic soda ash producers), the cost of transporting natural soda ash across
the country made it more economical for customers to buy soda ash from
synthetic producers. This economic
equation changed significantly in the early 1970s: in part because of higher
costs from increasingly stringent pollution control standards, but mostly
because of skyrocketing energy costs—and since energy was 50% of the cost of
producing synthetic soda ash, when energy costs soared, synthetic producers
could no longer compete with natural producers.
The result was that between 1972 and 1975 four of the eight major
synthetic soda ash plants in the country had already closed down.
In 1976 Diamond Shamrock added its soda ash
plant to that group when it announced in June that it was closing the entire
Painesville Works at the end of the year.
Though the facility made other chemicals, there were several factors
that added up to closing the entire Works:
a) the soda ash
produced at the plant was not all shipped to customers: 25% was used internally
at the Works to manufacture many other chemicals, which meant that closing down
the soda ash unit consequently ended the operations of other units
b) the same factors
that adversely affected the manufacture of soda ash—the high costs of both
energy and environmental controls—also adversely affected the Works overall
c) the Works was an old
facility (presumably in comparison to the company's other, newer industrial
chemical plants such as Battleground in Houston)
The closing of the
Painesville Works was indicative of the new direction Diamond Shamrock was
heading, for in 1979 the company announced its intention to become an energy
company rather than a chemical company.
In a move that demonstrated this new purpose, Diamond Shamrock's
headquarters was moved from Cleveland to Dallas later that year. Over the next several years the company
divested itself of non-energy divisions while continuing to acquire coal and
petroleum companies. The ultimate
divestiture occurred in 1986 when the company sold its all of its remaining
chemical business units to Occidental Petroleum. The following year the energy production and
exploration divisions of the company were split off to form Maxus Energy, and
Diamond Shamrock became strictly a petroleum refining and marketing company.
Over the next decade
Diamond Shamrock continued to grow and have success in petroleum refining and
retail petroleum sales. In 1996, the
company merged with Ultramar to become Ultramar Diamond Shamrock (UDS); however,
after this merger the company suffered one financial setback after another due
to the oil price crash of the late 1990s.
In 2001, UDS was bought out by Valero Energy, and the Diamond Shamrock
name disappeared into the history books.
The Diamond Story
A few years ago I was fortunate to
win an auction on eBay for a booklet about the Diamond's Painesville Works
called The Diamond Story at Painesville.
There is no author (though there is an introduction by M.O. Kirp, Works
Manager), and there is no date in it.
However, from reading through it and comparing certain facts with the
general histories referenced above, I have figured out that it is from
1956. When I showed this booklet to my
mother (her father—my grandfather, Bruce Merrill—worked as a millwright at the
Diamond from 1938 to 1975), she said that this was probably the booklet that
was used when the Diamond did a large-scale public open house when she was a
young girl.
The
booklet is full of information about the Works overall as well as the specific
manufacturing processes of different units in the Works—much of which I am
sharing here in transcription form (unfortunately, because of webspace
limitations, I cannot display scans of the entire booklet).
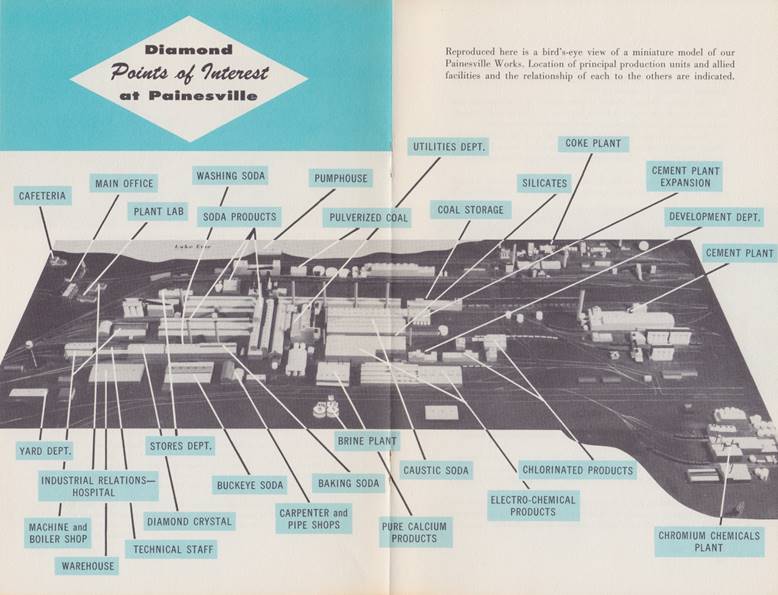
Before reading through
the transcribed information, let me draw your attention to the picture below:
this is a picture of a model of the Works that takes up the 'center spread' of
the booklet. This picture will be handy
to refer back to when reading through all the information I will be sharing on
this webpage from this point forward.
The only major facility that the picture does not show is the Diamond
Magnesium Plant, which was located further east on Fairport-Nursery Road (the
road depicted on the bottom/southern edge of the plant). By the way, this model still exists and is
being kept at the Fairport Harbor Marine Museum & Lighthouse.
Click on the image to view
a larger version.
Why Painesville Was Selected as Plant Site
Diamond's
first plant was built in 1912 at Painesville, on the southern shore of Lake
Erie, about 30 miles east of Cleveland.
Of the many sites studied for the new venture, this area was finally
selected in the belief the combination of its natural resources and man-made
advantages provided an ideal location with respect to supplying soda ash to the
glass industry, whose plants were then concentrated in western Pennsylvania,
southeastern Ohio and northern West Virginia.
The
area's attributes included among others:
1. A virtually
inexhaustible supply of salt—a strata about 500 feet thick and between 2,000
and 2,500 feet below the surface;
2. Availability of water
from Lake Erie, in unlimited quantity, for both product-processing and
equipment-cooling purposes, and
3.
Dependable,
economical transportation, via water and rail routes, for both delivery of
limestone from Michigan and coal from southern Ohio and West Virginia to the
plant, and shipment of finished products to consuming markets.
Here
in Painesville, then, four workhorses of modern applied industrial chemistry's
workaday world—salt, limestone, coal, and water—could be effectively harnessed
and products derived from them economically assembled.
Indeed,
this location proved to be a fortunate selection. Developments of the past quarter-century have
confirmed the wisdom of our founders' choice in many ways. Most important, aside from affording easy
access to these essential materials for alkali production, Painesville has
enabled Diamond to distribute, economically from one location, to the tri-state
area of Ohio, Pennsylvania and West Virginia; to the chemical orbit in the New
York-New Jersey-Philadelphia-Baltimore section, and to chemical plants in the
Midwest, particularly such centers as St. Louis, Cincinnati and Chicago.
Production
of soda ash started early in 1912, and customer demand proved so strong that
within three years capacity was increased to 800 tons daily. A portion of the new capacity, however, was
installed for caustic soda manufacture, launched in 1915. During World War I, in response to Government
request, Diamond doubled its caustic soda production early in 1918. The Company also embarked upon the
manufacture of bicarbonate of soda the same year.
Integration Proved Prime Consideration in
Early Days
Because
Diamond's founders were enamoured of the chemical industry's growth
possibilities, their long-term aim was to build an integrated operation not
only for producing the so-called "basic alkalies"—soda ash, caustic
soda and bicarbonate of soda—but also for utilizing a portion of them for
further processing and "upgrading" into other versatile chemicals.
This
expanding perimeter of new products thus called for new facilities and new
ideas; both followed at a steady pace during the next quarter-century, with
diversification and expansion sparked principally by more efficient and broader
usage of raw materials.
So,
in 1924, coke ovens were installed and an accompanying by-product plant built
to recover ammonia, gas and tar distillates.
Gas is used for fuel, ammonia and coke are required for soda ash. Benzene, toluene and related hydrocarbons are
derived from the tar distillates. This
installation enabled Diamond (1) to overcome a then-constantly-recurring
shortage of coke (used in soda ash production) and (2) further broaden its
product picture by the addition of premium-grade coke for foundries.
In
1925, facilities were established for producing precipitated, free-flowing
calcium carbonate of exceptionally high purity, a co-product of caustic soda by
the old lime-soda process. In 1937,
these facilities were further expanded.
Waste
limestone screenings not adaptable for soda ash manufacture led in 1925 to the
construction of a Portland cement plant.
Today, it is the leading factor in Northern Ohio's cement industry.
In
1926, an operation for packaging sal soda, lye and baking soda for household
use was organized.
Two
important developments brought this initial integration era in Diamond's
history to a close: manufacture of chlorine and production of bichromate of
soda. Because of Diamond's own salt beds
and power-generating facilities at Painesville, chlorine production was a natural
corollary development. Accordingly, and
electrolytic plant was put "on stream" in 1929. Its capacity has since undergone a number of
sizable expansions.
Soda
ash is an essential raw material for sodium bichromate; hence, it was equally
logical in this instance for Diamond to become interested in producing this
chemical. Production was consequently
started in 1931.
Other
significant growth moves followed in the ensuing years prior to World War
II. They included the manufacture of
carbon tetrachloride (a chlorine-derived solvent), and the development of
specialized alkaline detergents for use in the dairy and bottling industries as
well as in laundries.
Two-Fold War Production Job Accomplished
As
you might expect, Diamond contributed in diverse ways to the nation's military
production program during the war period.
At Painesville, this effort involved the manufacture of magnesium metal
for aircraft production, and the development and manufacture of "Chlorowax,"
a chlorinated paraffin.
Early
in 1941, when national defense quickly became "the order of the day"
along the industrial front, the Government requested Diamond to make metallic
magnesium. Because this operation
entails electrolytic decomposition of magnesium chloride, Diamond found it
necessary to develop a method for producing this material.
Diamond
engineers accomplished this objective through adapting certain portions of the
basic alkali process and applying these adaptations to the treatment of
dolomitic limestone. As Diamond was
getting ready to take over this production assignment, the Defense Plant
Corporation constructed a plant adjacent to our Painesville Works. Completed and put into operation in
September, 1942, this defense production facility soon exceeded its rated
capacity.
Operated
by Diamond through the Diamond Magnesium Company, an affiliate organized
specifically to carry out this mission, this plant remained in operation until
late 1945, long after other wartime magnesium production facilities had closed
down. (It received the Army-Navy
"E" Award for "excellence in war production.") When the Korean crisis came in 1951, the
Diamond Magnesium Plant was reactivated at Government request, and remained in
operation until mid-1953, when it again was "mothballed"; it is now maintained
as a stand-by facility for future defense needs.
Chlorowax,
a synthetic resin made from paraffin wax and chlorine, is an original Diamond
research development. Almost immediately
after its introduction, the material became widely used in the manufacture of
fire-retardant paints for application aboard Navy vessels, in the production of
tracer bullets, and in formulating flame-resistant compounds for impregnating
military textiles, such as camouflage nets, tents, fabrics, etc.
What We Make at Painesville …
Soda Ash
Production of soda
ash comprises a major operation of the Painesville Plant. To make this versatile basic chemical,
limestone from Michigan and coke from our own Coke Plant are mixed in proper
proportions and charged into large kilns to produce carbon dioxide gas and
lime. Salt is recovered as a solution
from a nearby deposit some 2,000 feet below ground level.
This solution, or
brine, is purified, saturated with ammonia gas, then carbonated in towers with
the gas recovered from the lime kilns. A
slurry leaving the bottom of the carbonating towers contains ammonium chloride
in solution and sodium bicarbonate as a solid.
The solid crude "bicarb" is then separated from the ammonium
chloride solution on rotary vacuum filters.
The ammonium
chloride solution is pumped to distillation columns, or lime stills, where the
lime, as a calcium hydroxide slurry, reacts with the ammonium chloride solution
to form ammonia gas and a solution of calcium chloride, a waste material. The recovered ammonia from the stills is
re-cycled to the absorbers to saturate more of the purified brine.
Washed and
filtered crude "bicarb" is decomposed in rotary dryers to produce
light soda ash, much of which is sold as a basic raw material to many
industries.
Part of the light
soda ash is processed into dense ash, primarily for the glass industry. Soda ash constitutes one of this industry's
most important raw materials; it combines chemically with sand to become molten
glass, from which a host of products are made.
This Diamond Chemical is also used in processing pulp and paper, iron
and steel, textiles, soap, and hundreds of other products.
Click
here to view a
diagram of the production process.
Chlorine and Caustic Soda
Chlorine and
caustic soda are derived from salt by a method most commonly referred to as
"the electrolytic process"—passage of electric current through a salt
brine solution, decomposing it into gaseous chlorine, caustic soda, and
hydrogen.
Principal raw
materials used are purified brine and electric power. The sodium chloride solution, after
purification, is decomposed in electrolytic cells using direct current. Resultant products are chlorine gas, a weak
caustic solution, and hydrogen.
The caustic
solution is concentrated, settled and filtered to remove salt and other
impurities. Hydrogen gas from the cells
is cooled and either used as a fuel or combined with chlorine to produce
anhydrous hydrochloric acid and muriatic acid.
(See the diagram in Chlorinated Products.) The chlorine gas is cooled, carefully dried
to remove water vapor, compressed, then piped into another section of the plant
for use in chlorination processing operations.
Dry chlorine gas
can be changed into a liquid by further cooling, compressing and
refrigerating. This liquid chlorine is
sold for use in water purification and sewage treatment, and to chemical plants
which use it as a chemical intermediate.
Dried liquid
chlorine is packaged in various containers to meet diverse industrial
demands. Some is put into 100-pound and
150-pound cylinders. Some is loaded in
ton containers shipped either by multi-unit tank cars in lots of 15 per car, or
by tractor-trailer trucks capable of carrying as many as 10 containers. For customers using larger quantities, liquid
chlorine is delivered in single-unit tank cars with capacities of 16, 30, or 55
tons.
Safety and careful
control characterize Diamond chlorine production, loading and
distribution. Control instrumentation is
an effective aid in improving safety, quality and efficiency of production.
Click
here to view a
diagram of the production process.
Chlorinated Products
Chlorinated products
made by Diamond at Painesville are Chlorowax, carbon tetrachloride, and
anhydrous hydrochloric acid.
To produce
Chlorowax, chlorine gas is reacted with paraffin, yielding grades containing
from 40 to 70 per cent of chlorine and ranging from liquids to resinous solids.
Carbon
tetrachloride is produced from chlorine gas and carbon bisulphide. Solvent blends, also made here, are used as
grain fumigants, fire extinguisher fluids, and for special purposes.
Chlorine and
hydrogen gas are used to produce a water solution of hydrogen chloride
(muriatic acid). The dissolved hydrogen
chloride is then stripped from the muriatic acid under pressure, refrigerated
and dried to produce a pure, dry hydrogen chloride gas.
Click
here to view
a diagram of the production process.
Baking Soda and Diamond Crystals
Soda ash is the
starting point in producing baking soda and Diamond Crystals.
In making baking
soda, a solution of soda ash is first filtered to remove insoluble matter, then
fed at a continuous rate counter-currently to a tower, through which carbon
dioxide gas is passing. The combination
of soda ash and carbon dioxide forms a "purified" sodium bicarbonate,
which is separated as a solid from the solution in high-speed centrifuges.
After centrifugal
washing, refined bicarbonate passes through dryers to remove the moisture, then
screened to yield a series of baking sodas for the food industry and related
fields. In recent years, the very-fine-particle
size baking soda has found a new market as the principal ingredient of a
dry-type fire extinguisher.
Diamond Crystals,
like baking soda, also rely on light soda ash as the basic raw material. The ash solution is filtered to remove
insolubles, then evaporated under controlled conditions to yield a crystalline
product, which is separated from the "mother liquor" in a centrifuge.
The crystals are
then washed, dried, and screened before packaging and shipment to many
detergent manufacturers, who use Diamond Crystals as a raw material in
formulating numerous washing and cleaning compounds.
Click
here to view a
diagram of the production process.
Silicate-Detergents
Diamond also
blends alkalies and detergents to produce washing soda and a wide variety of
other alkali cleansers required for specialized applications by our
customers. Also produced here are two
types of metasilicate—crystalline and anhydrous.
The first is
processed from liquid caustic soda and sodium silicate, the second from dense
soda ash and high-grade silica sand.
Both types find wide use in compounding detergents for application in
dairy and bottling plants, for metal-cleaning, and in commercial laundries.
Sal soda, extensively
used for water-softening purposes and as a household cleaner, is also produced
at Painesville. Still other Diamond
products made here are drain pipe opener, bowl cleaner and detergent compounds.
Click
here to view a
diagram of the production process.
Calcium Carbonates
From by-product
raw materials, Diamond produces calcium carbonate in many forms for the paint,
plastics, rubber and printing ink industries among others.
While the flow
diagram appears to be relatively simple, it is somewhat deceiving in that it
fails to indicate the exacting conditions and constant control which must be
maintained to perform the operations required in producing these chemicals. Raw materials for the most part are
by-products of our soda ash operations.
This part of the
Painesville Works, known to Diamond folks and customers alike as the "Pure
Calcium Products" Plant, has won national renown for the qualities of
purity and uniformity.
Click
here to view a
diagram of the production process.
Chromates
Soda ash and
chrome ore are the chief raw materials used to produce sodium bichromate.
Chrome ore
(imported from Africa mostly) is dried and crushed before entering a mixer,
where soda ash and dolomitic lime are blended with the pulverized ore in exact
ratios. This dry mixture is then charged
into oil or gas-fired rotary kilns, where the chrome compound fuses at high
temperature into a clinker.
Upon cooling, the
clinker is leached with water to remove the soluble chrome salts. Classifiers and filters then separate the
residue from the solution, which is next neutralized with sulphuric acid. A strong bichromate liquor is recovered
through filtration and evaporation.
A considerable
quantity of bichromate is sold as a liquid, but some portion of the liquor is
crystallized to produce bichromate crystals.
After drying and screening, they are sold in dry crystalline form.
Many operations
are required to purify the chromate solution, resulting in the production of
by-product chemicals in sufficient tonnage for sale in the industrial chemicals
market. These operations are carefully
controlled from the initial step of pulverizing the chrome ore through the
final stage of preparing the products for shipment.
While the
bichromate liquor is being purified, the first filtering of the original
solution produces aluminum hydroxide; and when the liquor passes through
salt-removing evaporators, these salts are fed through filters and dryers to
produce sodium sulphate.
Chromic Acid is
another chemical produced here. The
finished product, made from sodium bichromate and sulphuric acid, is sold in
flake form. Chromic Acid has many uses
throughout industry, the most important one being electroplating for both
decorative and protective purposes.
The Chromate Plant
at Painesville, one of the largest bichromate-producing facilities in America,
has been completely rebuilt in recent years.
It now incorporates processing equipment of the latest design, together
with facilities providing vastly improved working conditions.
Click
here to view a
diagram of the production process.
Cement
Principal raw
material used by the Standard Portland Cement Plant at Painesville consists of
limestone fines transported from upper Michigan to Fairport, and fines removed
by screening limestone at the Stone Dock.
They are transported by rail to the Cement Plant, where the stone is
pulverized in ball mills with clay harvested from clay pits adjoining the plant
property.
The slurry of
limestone and clay is then stored in large tanks and carefully adjusted to
proper ratios. It is then fed into
rotary cement kilns by two methods—de-watering it by filtering, and by direct
slurry feed.
With kilns fired
at high temperature and using pulverized coal, the limestone and clay decompose
and combine to produce a cement clinker, which is then cooled, pulverized in
ball mills with gypsum, and carefully classified to remove all coarse
particles.
The
finely-pulverized clinker is then pumped as a dry powder into the silos, where
it is stored in batch lots prior to analysis and testing to make certain the
finished product meets customer specifications.
Exacting methods
of chemical control are used extensively in the cement industry and the plant
here is no exception. Control over each
step of the operation is a basic requisite in meeting the high standards set by
the industry.
Production
capacity at the plant has been steadily expanded in recent years. In 1954, for example, capacity was increased
by 380,000 barrels through the conversion of a lime-burning kiln to cement
manufacture. The following year an
additional 320,000 barrels were made possible with the installation of a new
finished cement grinding mill with auxiliary equipment.
Presently under
way is another project which, when completed, will increase capacity by another
800,000 barrels. Thus, within a
three-year period, capacity will have been increased from 1,200,000 barrels to
2,700,000 barrels.
Click
here to view a
diagram of the production process.
Coke and Coke By-Products
Primary function
of the Coke Plant is to produce a high-grade, properly-sized coke for burning
limestone used in soda ash manufacture. Surplus
coke is prepared for and sold to the foundry industry. Coke oven gas, a by-product, is used by other
production units at our Painesville Works.
Several grades of
coal, rail-transported from Pennsylvania and West Virginia, are properly
pulverized and proportioned for charging into the coke ovens. Coal is heated with coke oven gas already
produced until all volatile matter is driven from the coal into collection
headers. Next, the hot coke is pushed
from the oven, quenched with water, screened to various required sizes, then
loaded into hopper cars for shipment to the lime kilns or to our customers.
Recovered coke
oven gas is first scrubbed with water to remove ammonia and water-soluble
salts, then passed through oil scrubbers, which absorb benzene, toluene and
xylene. Finally, the gas flows through
layers of wood chips, saturated with iron oxide. This operation removes sulphur compounds from
the gas. Now purified, it is distributed
throughout the plant for use as a fuel.
Water and wash-oil
from the gas scrubbers are recovered and stripped in steam stills to recover
the ammonia, sulphides, benzene, toluene and xylene.
Click
here to view a
diagram of the production process.
Plant Consumes Raw Materials in Huge
Quantities
A few statistics may help you to
better visualize the scope of our operations at Painesville. In a single day we use
* 100 million
gallons of water, enough to more than meet the daily requirements of the City
of Cleveland.
* 2,300 tons of limestone, enough to fill 40
fifty-ton freight cars.
* 1,000 tons of coal, enough to supply 225
homes for a year.
*
3,000 tons of salt, enough to supply the yearly needs of 650,000
families.
In
addition to these basic raw materials, suppliers from many parts of the United
States and elsewhere send us other raw materials required to operate our
plant. They include silica sand,
limestone fines, gypsum, and chromite ore.
We supply our own clay, salt and purified brine.
Chronology of the
Painesville Works
(A
key to sources is listed at the bottom of this section)
The Beginning and
The End
Diamond
Alkali began operating in 1912 [1]
The
Chromate Plant was shut down in 1972 [1]
The
majority of the Works was shut down in 1976
[1]
The
last remaining operating unit of the Works, the Chlorowax Plant, was shut down
in 1977 [1]
Below
are details for specific plants within the Works:
Main Plant
(On
map above: Soda Products, Washing Soda, Diamond Crystal, Buckeye Soda,
Baking
Soda, Caustic Soda, Pure Calcium Products)
Soda
Ash began operations 1912 [D]
Caustic
Soda began operations 1915 [D]
Bicarbonate
Soda began operations 1918 [D]
ceased
operations 1976 [1]
sold
for scrapping/demolition 1978 [1]
Chlor Alkali Plant
(On map above: Silicates, Electro-Chemical
Products)
built
1929, began operations 1929 or 1930 [D,1]
ceased
operations 1976 [1]
sold
for scrapping/demolition 1978 [1]
Hydrochloric Acid Plant
(On map above: Chlorinated Products)
began
operations 1930 [1]
ceased
operations 1976 [1]
sold
for scrapping/demolition 1978 [1]
Carbon Tetrachloride Plant
(On map above: Chlorinated Products)
built/began
operations 1933 [1]
ceased
operations 1976 [1]
sold
for scrapping/demolition 1978 [1]
Chlorowax Plant
(On map above: Chlorinated Products)
built/began
operations 1944 [1]
ceased
operations 1977 [1]
sold
for scrapping/demolition 1978 [1]
Coke Plant
built/began
operations with 1 battery of 23 Koppers-Becker ovens 1924 [D,P,M,1,2]
additional
battery of 23 Koppers-Becker ovens built/began operations circa 1937 [M,K]
sold
to Erie Coke & Chemical Co. 1976 [1,2]
ceased
operations 1982 [1,2]
sold
for demolition 1983 [1,2]
demolished
1986-1988 [1,2]
Standard Portland
Cement Plant
PLANT
A
(On
map above: Cement Plant)
built/began
operations 1924 or 1925 [D,1,3]
closed
1961 [3]
structures
used for cement storage until 1968 [3,T]
structures
left idle until 1980 [conjecture]
sold
to Aluminum Smelting & Refining Co. 1980
[4]
leased
to Cousins, Inc. 1992 [4]
sold
to Cousins, Inc. 1997 [4]
sold
for demolition 2004 [4]
demolished
2005-2006 [based on 2006 aerial
photos]
PLANT B (On map above: Cement Plant
Expansion; converted portion of Caustic Soda building)
began
operations 1954 [D]
closed
1964 [3]
structure
left idle until 1978 [conjecture]
sold
for scrapping/demolition 1978 [as part of Caustic
Soda building]
Chromate Plant
(On map above: Chromium Chemicals Plant)
built/began
operations 1931 [1,D]
ceased
operations 1972 [1]
plant
demolished 1972-1973 [based on 1973 aerial
photo]
area
sealed 1978-1980 [1]
Magnesium Plant
(Not on map above: located east of Painesville
Works at 720 Fairport-Nursery Road [5])
built/began
operations 1942 [D,6]
deactivated
1945 [D]
reactivated
1951 [D]
deactivated
1953 [D,6]
sold
in two parts 1963 [5]
Western 2/3 Portion
sold
to US Rubber (Uniroyal) 1963 [5]
ceased
operations 1999 [6,7]
subsequently
demolished [based on 2002 aerial
photo]
Eastern 1/3 Portion
sold
to Pillsbury 1963 [L:11B-040OLD]
sold
to Glyco 1969 [8]
Glyco
merged into Lonza 1986 [9,10]
sold
to Twin Rivers Technologies 2002 [9,10]
Another
industry located adjacent to the Painesville Works but that was never part of
Diamond Alkali/Shamrock will be included here since US Rubber (Uniroyal)—who
bought part of the Diamond facility above—at one time owned this facility as
well:
Glenn L. Martin Chemical Company
(Not on map above: located on eastern edge of
Diamond property at 900 Fairport-Nursery Road [11])
built/began
operations 1947 [11]
sold
to US Rubber (Uniroyal) 1949 [7,11]
ceased
operations 1975 [11]
sold
to Dart Cartage (Dartron) 1979 [11]
sold
to Crompton Manufacturing 2001 [L:12A-051]
Sources
1 Ohio EPA DSPW Site: Director's Final
Findings and Orders, 9/27/95
2 Ohio EPA DSPW OU6 Site: Director's
Final Findings and Orders, 7/13/06
3 FTC Docket 8572, 72 FTC 700: In the
Matter of Diamond Alkali Company, 10/2/67
4 Ohio EPA DSPW OU2 Site: Director's
Final Findings and Orders, 7/13/06
5 US Army Corps of Engineers Painesville
Site: Engineering Valuation/Cost Analysis, June 1998
6 US Army Corps of Engineers Painesville
Site: Final Record of Decision, April 2006
7 Ohio EPA Uniroyal Site: Director's
Final Finding and Orders, 5/14/99
8 "Glyco Buys Pillsbury
Plant," Soap & Chemical Specialties, March 1969
9 "Lonza Exits Fatty Acid
Manufacturing With Plant Sale," Chemical Market Reporter, 9/16/02
10 "Lonza Sales [sic] its Painesville
Fatty Acids and Glycerine Plant," Lonza Press Release, 1/7/03
11 Dartron Corp v Uniroyal Chemical Co.,
893 F.Supp. 730, 1995
D The Diamond Story at Painesville, 1956
P Trade Publications: American Gas
Association Fifth Annual Convention Proceedings, 1923
and Gas Industry, Vol.
24, 1924
M Fairport, Painesville & Eastern
Valuation Maps
K Koppers-Becker Coke Ovens, 1944
T Testimony from ICC Docket 23980
L Lake County Tax Map
Sources
1, 2, 4, and 7 are available for free download on the Ohio EPA website here.
Sources
5 and 6 are available for free download on the ACE website here.
Sources
3, 11, P, and K can be found at any large/major library.
Sources
8-10 can be found on the internet.
Source
D is discussed above; I am not sure where any other copies can be obtained.
Sources
M and T are available from the National Archives (as discussed on my FP&E Resources
page).
Source
L is available on the Lake County website (as discussed on my FP&E Maps page).
Notes and Observations
I have posted some aerial photos of the Diamond's
facilities on my flickr account; click here
to view them.
As per references in
numerous ICC documents, the FP&E did not provide intra-plant switching
services for the Diamond. However, that
statement does not mean that much: Basically, due to the nature of the
Diamond's product shipments and the layout of the Painesville Works, for the
most part the only activity that was necessary on the Diamond's tracks was the
spotting of empty or loaded cars—and any switching that had to take place to
get cars ready for spotting was handled by the FP&E on their yard
tracks. Even in cases where cars were
unloaded at one part of the plant and then taken to another part of the plant
to be loaded (and vice-versa), such switching moves were deemed FP&E
intrastation moves (the station being "Alkali"). But though the FP&E handled most of the
switching duties that needed to take place for the Diamond, the Works still had
a need to move cars within the confines of its facilities, and through the
years they used various small industrial locomotives for those duties—such as a
Plymouth 30-tonner, a couple GE 25-tonners, and a couple GE 45-tonners. One of the 45-ton units was used to move the
quench car at the Coke Plant, and in this photo
from 1951 (a portion from one of the aerial photos I refer to above), you can just
make out the GE switcher moving the quench car between the coke ovens and the
coke wharf (at the base of the large smoke stack in the center of the
photo). Another 45-tonner in a nice
green-and-yellow paint scheme was used to move FP&E hoppers at the
Diamond's Stone Dock. A close-up picture
of that switcher in the FP&E's West Yard can be viewed here; and a more
recent picture of the same switcher—albeit, permanently out of commission—can
be viewed here.
In the book Trackside
Around Eastern Ohio (more about this book near the bottom of this
page), on page 59 there is a photo caption that reads, in part: "The
limestone came off the lake at the dock and was used in the making of coke at
the Diamond Alkali plant …." As can
be seen from the information above, this is incorrect: the limestone was used
primarily for making soda ash, and secondarily for making cement. On page 60 of the same book there is a photo
with an accompanying caption that reads, in part: "Diamond Alkali … was a
major producer of coke for the steel industry.
Here Dave catches #104 and #107 making up a drag for the N&W at
Perry. The Diamond Shamrock chemical
plant closed in the 1970's dealing a blow to the FP&E but the coke plant
remained into the 1990's …." For
the following reasons, these statements are incorrect:
1) As explained in "The Diamond
Story" section above, the Diamond was a producer of coke primarily for
itself; and though it did sell surplus coke to outside customers, from the
materials I have read the Diamond would not be considered a major
producer. The Diamond produced
"furnace coke," and in 1973 when the Coke Plant was running at peak
capacity (the previous year both coke batteries had been rebuilt) it produced
about 680 tons of coke per day—of which about 200 tons went to the lime kilns,
leaving roughly 480 tons available to be shipped to outside customers each day. When the Diamond sold the Coke Plant to Erie
Coke & Chemical, the new owner produced "foundry coke" which,
because of the longer coking time required, reduced the plant's total
production amount to about 425 tons of coke per day. These daily production amounts may sound like
a lot, but when compared to a true major coke producer such as the US Steel
Clairton Coke Works, which in 1980 produced more than 16,000 tons of coke per
day (and in the 1940s and 1950s produced 21,000 tons of coke per day), then the
Diamond's Coke Plant was definitely a minor producer.
2) In the photo the caption refers to, it is
implied that the hoppers in the photo are going to be interchanged with the
N&W for shipment to a customer.
However, the cars in the photo are the FP&E's 800-series hoppers,
which, as I discuss on my FP&E
Freight Car Roster page, were limited to use on FP&E rails only—meaning
that the hoppers in this photo are actually in the midst of an intrastation
move of coke from the Coke Plant (on the east side of the Painesville Works) to
the lime kilns (on the west side of the Painesville Works).
3) As shown in the Chronology section of this
page, the Coke Plant ceased operations in 1982.
Created
by Scott Nixon
July
2009
Updated: October 2010, April 2011, September
2021